OncoLaB
OncoLaB
A New Wave of
Therapeutic Platform Innovation
Therapeutic Platform Innovation
온코랩채용가이드
OncoLaBHiring Guide
Contact Us
OncoLaB
A New Wave of
Therapeutic Platform Innovation
Therapeutic Platform Innovation
-
2025.12.19
ANGel
Combination immuno oncology—built through formulation
ANGel is a hydrogel microparticle based precision formulation and delivery platform. Designed for peritumoral / perilesional subcutaneous (SC) administration, ANGel connects three elements into one platform logic: localized exposure design, a sustained exposure profile, and modular combination architecture.
At its core, ANGel is built to work with approved antibodies and small molecule oncology assets—without molecular redesign—to express bispecific inspired multi mechanism and ADC like payload action at the formulation level.
At its core, ANGel is built to work with approved antibodies and small molecule oncology assets—without molecular redesign—to express bispecific inspired multi mechanism and ADC like payload action at the formulation level.
ANGel
Novel Therapeutic
Platform
Platform
Hydrogel
submicron-scale
microparticle
submicron-scale
microparticle
-
Delivery
Local, peritumoral precision delivery by design
-
Efficacy
Modular architecture for multi antibody combinations and rational combinations
-
Sustainability
Sustained exposure engineered around local tissue residence
What makes ANGel different
-
Local precision delivery
ANGel makes local exposure control the starting point—not an afterthought. - Concentrates exposure where it matters
- Designed to reduce unnecessary systemic exposure burden
- Built with co delivery / co activation in mind for combination therapy
-
Combination by design
Combination therapy shouldn’t be “added on”—it should be architected from day one. - Supports multi antibody combinations and modular combination configurations
- Enables mechanism driven strategies (e.g., addressing immune escape)
- Structured for fast validation and scalable expansion
-
Sustained exposure profile
ANGel is engineered around how long it stays where it’s delivered. - Sustained local exposure profile informed by subcutaneous tissue residence
- Designed with reduced dosing frequency as a development direction
- Built to support patient friendly treatment concepts
Existing subcutaneous injection

systemic diffusion
 +
+
 =
=
 BsAb
BsAb
 +
+
 =
=
 ADC
ADC
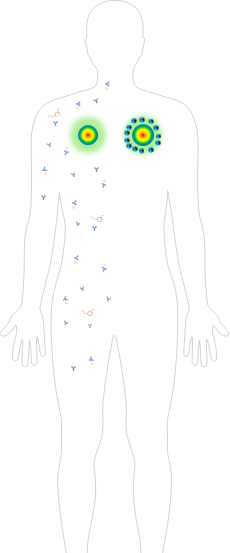
Beyond systemic diffusion

- Various modules of antibodies small molecules
- Precision SC delivery
- Long half-life
- No cytokine & systemic toxicity
Easy manufacture & increased efficacy
No risk of sudden burst drug release
Spec driven and tech transfer ready (CQA defined)
Platform positioning
| OncoLab | J Corp | L Corp | A Corp | |
|---|---|---|---|---|
| Product | ANGel | Bispecific Antibody | Antibody-drug conjugate | Hyaluronidase |
| Administration Method |
Subcutaneous | Intravenous | Intravenous | Subcutaneous |
| Efficacy for TNBC (mOS) |
>3× improvement (pre-clinical) |
30 mo (Phase II) | 24 mo (Phase II) | 18 mo (Approved) |
| Clinical Entry | ~3 years | ~10 years | ~10 years | ~3 years |
| Platform Scalability | Very high (Use of approved drug) |
Low (Developing almost new antibody |
Low (Complex conjugation processes |
Very high (Use of approved drug) |
Why it matters
- Targeted exposure: engineer where exposure happens
- Personalized combinations: configure combinations by indication and mechanism
- Sustained profile: design for persistence at the delivery site
- Reformulation advantage: develop on validated assets to reduce risk
- Scalable platform: expand across combinations and indications with a modular approach